| ethylenediaminetetraacetic acid (EDTA) |
Sunday, December 28, 2014
EDTA
A chelating agent of particular economic significance is ethylenediaminetetraacetic acid (EDTA).
EDTA is a versatile chelating agent. It can form four or six bonds with a metal ion, and it forms chelates with
both transition-metal ions and main-group ions. EDTA is frequently used in soaps and detergents, because it
forms a complexes with calcium and magnesium ions. These ions are in hard water and interfere with the
cleaning action of soaps and detergents. The EDTA binds to them, sequestering them and preventing their
interference. In the calcium complex, [Ca(EDTA)]2–, EDTA is a tetradentate ligand, and chelation involves
the two nitrogen atoms and two oxygen atoms in separate carboxyl (-COO–) groups. EDTA is also used
extensively as a stabilizing agent in the food industry. Food spoilage is often promoted by naturally-occurring
enzymes that contain transition-metal ions. These enzymes catalyze the chemical reactions that occur during
spoilage. EDTA deactivates these enzymes by removing the metal ions from them and forming stable chelates
with them. It promotes color retention in dried bananas, beans, chick peas, canned clams, pecan pie filling,
frozen potatoes, and canned shrimp. It improves flavor retention in canned carbonated beverages, salad
dressings, mayonnaise, margarine, and sauces. It inhibits rancidity in salad dressings, mayonnaise, sauces, and
sandwich spreads. EDTA salts are used in foods at levels ranging from 33 to 800 ppm.
Wednesday, June 4, 2014
Tuesday, March 25, 2014
Breathalyzer
Chemistry of a Breathalyzer
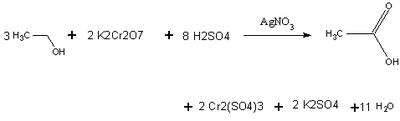
Using Beer's law, the spectrophotometer can relate concentration to absorbance levels of the chromium ion. The amount of alcohol present is proportional to the stoichiometric coefficients. An actual breathalyzer only needs to detect 25 micrograms of ethanol to give a reading 0.10 Blood Alcohol Level.
Credit : http://chem242.blogspot.com/2005/08/chemistry-of-breathalyzer.html
Sunday, March 23, 2014
Saturday, March 22, 2014
Monday, February 24, 2014
Expanded PS
Polystyrene Boxes for Food
Concerns
over the use of PS should best be analysed based on facts rather than on
perception .
The fact
that advanced countries like Japan , US and The EU continue to allow the use of
PS as a food packaging material speaks volume about its safety .
Basically there are 2 forms of PS foam
·
Extruded polystyrene [ foam plates , egg cartons and other food food service applications ]
·
Expanded polysterene [ coffee
cups , packaging of delicate E & E appliances
, consumer products ]
Both
types are also used as thermal insulation in Industrial , commercial and
residential construction.
[i]What is EPS?
EPS (Expanded Polystyrene) or as many know by The Dow Chemical Company's
trade marked name, STYROFOAM,
is an extremely lightweight product that is made of expanded polystyrene beads.
Originally discovered by Eduard Simon in 1839 in Germany by accident, EPS foam
is more than 95% air and only about 5% plastic.
Small solid plastic particles of polystyrene are made from the monomer
styrene. Polystyrene is normally a solid thermoplastic at room temperature that can be melted at
higher temperature and re-solidified for desired applications. The expanded
version of polystyrene is about forty times the volume of the original
polystyrene granule.
Expanded polystyrene (EPS) is a
versatile, lightweight material that can be manufactured into a variety of
products. EPS offers a high-performance
yet economical support for a wide variety of items—from sensitive
electronics to appliances to pharmaceuticals—to be safely delivered to market.
Manufacturers rely on EPS packaging because of its ability to prevent or minimize product damage during
transit and its excellent insulation properties required for food and
medical shipments.
 Yet
EPS packaging, just like any disposable packaging, will eventually become a solid waste and have to be managed. But here’s the good news: EPS
is recyclable. Although the availability of polystyrene recycling programs
varies by community and can be limited, the EPS industry uses average of 50
percent of the post consumer material collected in the manufacture of new EPS
transport packaging and loose fill packaging, which has reduced requirements
for raw material resources, energy consumption has diverted material from landfills.
Yet
EPS packaging, just like any disposable packaging, will eventually become a solid waste and have to be managed. But here’s the good news: EPS
is recyclable. Although the availability of polystyrene recycling programs
varies by community and can be limited, the EPS industry uses average of 50
percent of the post consumer material collected in the manufacture of new EPS
transport packaging and loose fill packaging, which has reduced requirements
for raw material resources, energy consumption has diverted material from landfills.
Subscribe to:
Comments (Atom)