Chemistry of a Breathalyzer
A Breathalyzer makes use of the fact that alcohols (in this case ethanol) oxidize into carboxylic acids. It uses the strong oxidizing agent Potassium dichromate in a yellow solution of sulfuric acid, under the presence of a Silver Nitrate catalyst, to complete the reaction quickly. As ethanol oxidizes and the Potassium dichromate reacts, the chromate ion changes from Cr (VI) to Cr (III). This causes the color intensity of the yellow solution to decrease, and a spectrophotometer in the breathalyzer compares the absorbance of this solution with that of an unreacted solution
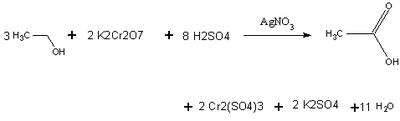
Using Beer's law, the spectrophotometer can relate concentration to absorbance levels of the chromium ion. The amount of alcohol present is proportional to the stoichiometric coefficients. An actual breathalyzer only needs to detect 25 micrograms of ethanol to give a reading 0.10 Blood Alcohol Level.
Credit : http://chem242.blogspot.com/2005/08/chemistry-of-breathalyzer.html
