| ethylenediaminetetraacetic acid (EDTA) |
Strictly Chemistry
Sunday, December 28, 2014
EDTA
A chelating agent of particular economic significance is ethylenediaminetetraacetic acid (EDTA).
EDTA is a versatile chelating agent. It can form four or six bonds with a metal ion, and it forms chelates with
both transition-metal ions and main-group ions. EDTA is frequently used in soaps and detergents, because it
forms a complexes with calcium and magnesium ions. These ions are in hard water and interfere with the
cleaning action of soaps and detergents. The EDTA binds to them, sequestering them and preventing their
interference. In the calcium complex, [Ca(EDTA)]2–, EDTA is a tetradentate ligand, and chelation involves
the two nitrogen atoms and two oxygen atoms in separate carboxyl (-COO–) groups. EDTA is also used
extensively as a stabilizing agent in the food industry. Food spoilage is often promoted by naturally-occurring
enzymes that contain transition-metal ions. These enzymes catalyze the chemical reactions that occur during
spoilage. EDTA deactivates these enzymes by removing the metal ions from them and forming stable chelates
with them. It promotes color retention in dried bananas, beans, chick peas, canned clams, pecan pie filling,
frozen potatoes, and canned shrimp. It improves flavor retention in canned carbonated beverages, salad
dressings, mayonnaise, margarine, and sauces. It inhibits rancidity in salad dressings, mayonnaise, sauces, and
sandwich spreads. EDTA salts are used in foods at levels ranging from 33 to 800 ppm.
Wednesday, June 4, 2014
Tuesday, March 25, 2014
Breathalyzer
Chemistry of a Breathalyzer
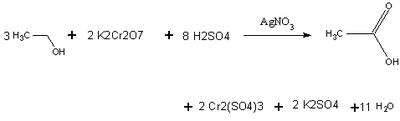
Using Beer's law, the spectrophotometer can relate concentration to absorbance levels of the chromium ion. The amount of alcohol present is proportional to the stoichiometric coefficients. An actual breathalyzer only needs to detect 25 micrograms of ethanol to give a reading 0.10 Blood Alcohol Level.
Credit : http://chem242.blogspot.com/2005/08/chemistry-of-breathalyzer.html
Sunday, March 23, 2014
Saturday, March 22, 2014
Subscribe to:
Comments (Atom)